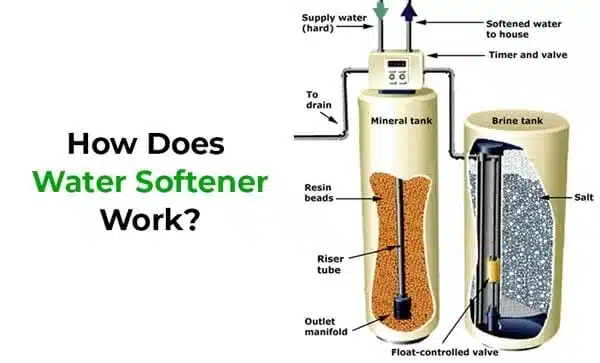
A water softener is a device designed to remove minerals like calcium and magnesium from hard water and replace them with sodium ions. This process is known as ion exchange. The purpose of using a water softener is to prevent the negative effects of hard water, such as scale buildup in pipes and appliances, reduced lathering of soaps, and the potential for clogs and corrosion.
Here’s how a typical water softener works:
-
Resin Tank: The main component of a water softener is the resin tank. Inside this tank, there are tiny resin beads or resin particles. These beads are typically made of polystyrene and are coated with a sodium solution.
-
Ion Exchange: When hard water enters the resin tank, the calcium and magnesium ions in the water are attracted to the resin beads due to their positive charge. The sodium ions on the resin beads are released into the water in exchange for the calcium and magnesium ions. This process essentially “softens” the water by removing the hardness-causing minerals.
-
Softening Cycle: As the resin beads become saturated with calcium and magnesium ions, the water softener initiates a regeneration cycle. This cycle involves flushing the resin beads with a brine solution (sodium chloride or salt water). The brine solution contains a high concentration of sodium ions.
-
Regeneration Process: The brine solution flows through the resin tank, and the high concentration of sodium ions in the solution displaces the calcium and magnesium ions on the resin beads. The calcium and magnesium ions are then carried away in the wastewater, leaving the resin beads ready to soften water again.
-
Rinsing and Flushing: After the regeneration process, the resin beads are rinsed to remove excess brine solution and any remaining traces of calcium and magnesium ions. This ensures that the softened water produced by the water softener does not have a high sodium content.
-
Ready for Use: Once the rinsing process is complete, the water softener is ready to soften water again. It continues this cycle of ion exchange, regeneration, and rinsing as needed based on the water usage and the capacity of the resin tank.
It’s important to note that while water softeners are effective at removing hardness-causing minerals, they do add a small amount of sodium to the water. This might be a concern for individuals on low-sodium diets. Additionally, some modern water softeners use alternative technologies that do not involve sodium exchange, such as using potassium chloride or other methods of ion exchange. These alternative methods are designed to address the sodium content concern while still effectively softening the water.